Meeting the requirements of the EU Falsified Medicines Directive
implementation of the EU Falsified Medicines Directive at Biokanol pharma
HSA Systems and Brainority deliver complete print, verification and serialisation solution to BIOKANOL Pharma in Rastatt, Germany. Thanks to this solution BIOKANOL Pharma complies with the EU Falsified Medicines Directive.
Fully automated print & verification unit from HSA Systems
Rastatt-based BIOKANOL Pharma is a specialist in secondary packaging for medicines and dietary supplements. In 2016, BIOKANOL Pharma decided to implement EU Directive 2011/62/EU (FMD) on falsified medicinal products with a target date of 9th February 2019. In order to comply with the EU Directive, the contract packaging industry is expanding into serialisation.
To implement the requirements of individually coding each piece of packaging, BIOKANOL selected a fully automated PV650C print and verification unit from HSA Systems. The generation, management and exchange of serial data is carried out by the Secident Pharma Coding System Software from Brainority Software GmbH based in Stuttgart.
The PV650C is a modular, compact station for direct printing with HP printing technology and a high-resolution camera system for evaluating, verifying and grading printed images
Direct printing of pharma codes on packaging
The PV650C is a modular, compact station for direct printing with HP printing technology and a high-resolution camera system for evaluating, verifying and grading printed images. The HSAJET® software provides country-specific coding formats, such as GS1 DataMatrix or China coding - additional formats can be retrofitted. The HSAJET® hardware enables the entire unit to be controlled from one interface.
The system solution was developed and manufactured entirely by HSA Systems in line with GAMP guidelines and offers full conformity with the pharmaceutical industry’s quality standards.

SERIALISATION WITH SECIDENT SOFTWARE
The Secident Pharma Coding System from Brainority Software manages and transfers encrypted serial numbers and production data (e.g. PPN, LOT, EXP) directly to the printing system. The machine operator can carry out the new printing job without any additional configuration of the software. After verifying and balancing the relevant codes, the serial numbers from Secident can be processed, either back to the client or direct to the securPharm database system or the European Hub.
Secident's software-as-a-service architecture means that no additional IT infrastructure or support is required for serialisation at BIOKANOL. Using the software at the data centre in Stuttgart satisfies all data exchange, hardware and maintenance costs and is certified by DQS according to ISO/IEC 27001.
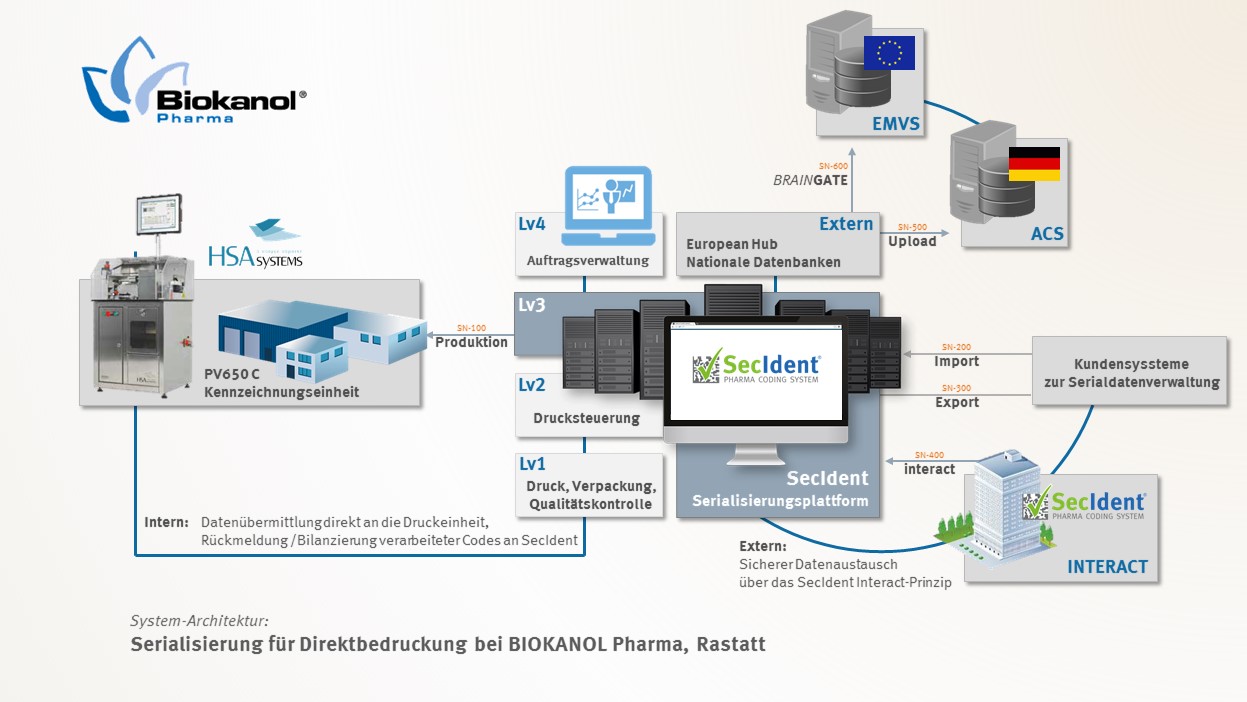
System architecture: Serialisation for direct printing at BIOKANOL Pharma

Print, Verification & Serialisation according to the EU Falsified Medicines Directive
Successful implementation at BIOKANOL Pharma
Thanks to the PV650C print & verification unit from HSA Systems and the Secident software from Brainority, BIOKANOL Pharma complies with the FMD.
The entire hardware and software solution was installed in 2016 and staff members were trained in using the systems. Using both the PV650C marking unit and the Secident software enabled the systems to be successfully validated. BIOKANOL’s scheduled guidelines for being able to apply the system for production from early 2018 were successfully implemented.
BIOKANOL Pharma is already setup for serialisation, a year ahead of the EU directive on falsified medicinal products coming into effect, and it has already been able to convince numerous clients about its individual coding service.

